Solvent-free continuous flow hydrogenation of N-methylpyrrolidone to N-methylpyrrolidine catalyzed by bimetallic Pt/V on HAP†
Abstract
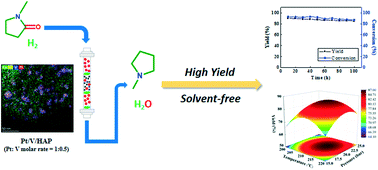
Herein, solvent-free continuous flow bimetallic Pt/V catalytic hydrogenation of N-methylpyrrolidone (NMP) to prepare N-methylpyrrolidine (NMPD) was developed. The yield of NMPD was as high as 89.41%, and the best weight hourly space velocity was 1.2 h−1 under the optimal reaction conditions, which were screened by the response surface methodology. The catalysts with different ratios of Pt and V supported on hydroxyapatite (HAP) were prepared and evaluated for activity for the hydrogenation of NMP. The best catalyst proportion was Pt/V = 1 : 0.5 on HAP, which was characterized by STEM-EDS, ICP-OES, BET, XPS, and PSD analyses. It was shown that polyvalent Pt and V were highly dispersed on HAP and the synergistic effect between Pt and V species contributed to the excellent catalytic performance. More importantly, the catalyst stability was excellent and after running for 100 h, the yield remained at 85.21%. Significantly, this solvent-free continuous flow catalytic hydrogenation of an amide provides a green, efficient, low-cost and environmentally friendly process for the synthesis of NMPD.


 Please wait while we load your content...
Please wait while we load your content...