Tracking the in situ generation of hetero-metal–metal bonds in phosphide electrocatalysts for electrocatalytic hydrogen evolution†
Abstract
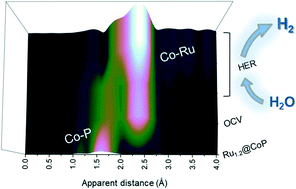
Transition metal phosphides (TMPs) have been intensively pursued in the field of hydrogen evolution reaction (HER) catalysts. The electrocatalytic performance of TMPs can be further improved by combining heteroatoms to manufacture bimetallic phosphides, which modifies the electronic structure and atomic configuration. To date, the knowledge of how synergistic effects could promote the high-efficiency catalyst at the atomic level is still largely inadequate. Herein, a Rux@CoP electrocatalyst was investigated to clarify the synergistic effect between Co sites and metallic Ru during the HER. Optimized Ru1.2@CoP exhibits the best catalytic activity in acidic solution, achieving a current density of 10 mA cm−2 at a low overpotential of 16 mV. Notably, by employing in situ X-ray scattering/diffraction and absorption spectroscopy, the generation of newly-formed Co–Ru bonds on Co sites is discovered during the HER. The in situ formation of the Co–Ru moiety suppresses the formation of metallic Co under HER conditions and dominates the catalytic behavior of bimetallic electrocatalysts.
- This article is part of the themed collection: In situ and operando spectroscopy in catalysis


 Please wait while we load your content...
Please wait while we load your content...