Competition between metal-catalysed electroreduction of dinitrogen, protons, and nitrogen oxides: a DFT perspective†
Abstract
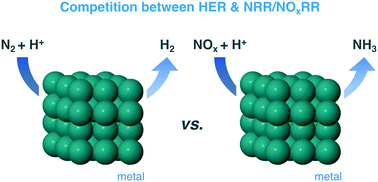
Production of green ammonia through electrochemical reduction of N2 is amongst the major challenges of applied catalysis today. A large number of materials have been reported to be capable of catalysing the nitrogen reduction reaction (NRR), but the reliability of these results has been questioned due to the lack of evidence for N2 being the source of produced NH3. In the present study, we use density functional theory (DFT) to demonstrate that the NRR is highly unfavoured versus the hydrogen evolution reaction (HER) on a wide selection of metal catalysts, some of which have been reported to be active for the N2 electroreduction to ammonia previously. Most importantly, we provide a comprehensive analysis of ammonia formation through electroreduction of nitrogen oxides, specifically NO and NO2, which are very hard to avoid as impurities in the NRR experiments, and demonstrate that these processes can effectively compete with the HER. In general, N2 weakly adsorbs on the metal surfaces but NO and NO2 exhibit stronger interactions and a greater stability of the reduced intermediates. These results highlight NO/NO2 reduction as a potential source of ammonia in NRR experiments and might also guide future design of catalysts for the fundamentally and practically important nitrogen oxide reduction reactions.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...