Highly efficient and selective aqueous phase hydrogenation of aryl ketones, aldehydes, furfural and levulinic acid and its ethyl ester catalyzed by phosphine oxide-decorated polymer immobilized ionic liquid-stabilized ruthenium nanoparticles†
Abstract
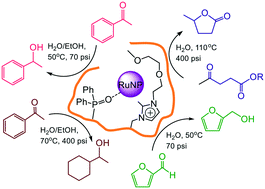
Impregnation of phosphine-decorated styrene-based polymer immobilized ionic liquid (PPh2-PIIL) with ruthenium(III) trichloride resulted in facile reduction of the ruthenium to afford Ru(II)-impregnated phosphine oxide-decorated PIIL (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh2PIIL). The derived phosphine oxide-decorated polymer immobilized ionic liquid-stabilized RuNPs (RuNP@O
PPh2PIIL). The derived phosphine oxide-decorated polymer immobilized ionic liquid-stabilized RuNPs (RuNP@O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh2-PIILS) catalyse the highly efficient and selective aqueous phase reduction of the carbonyl group in aryl and heteroaryl ketones and aldehydes, including furfural, as well as the hydrogenation of levulinic acid and its ethyl ester to afford γ-valerolactone (GVL). While this is the first report of RuNPs stabilized by a phosphine oxide-modified support, there appear to be several recent examples of similar serendipitous oxidations during the synthesis of RuNPs by impregnation of a phosphine-decorated polymer with ruthenium trichloride; as these were either misinterpreted or not recognised as such we have carried out a detailed characterization and evaluation of this system. Reassuringly, RuNP@O
PPh2-PIILS) catalyse the highly efficient and selective aqueous phase reduction of the carbonyl group in aryl and heteroaryl ketones and aldehydes, including furfural, as well as the hydrogenation of levulinic acid and its ethyl ester to afford γ-valerolactone (GVL). While this is the first report of RuNPs stabilized by a phosphine oxide-modified support, there appear to be several recent examples of similar serendipitous oxidations during the synthesis of RuNPs by impregnation of a phosphine-decorated polymer with ruthenium trichloride; as these were either misinterpreted or not recognised as such we have carried out a detailed characterization and evaluation of this system. Reassuringly, RuNP@O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh2-PIILS generated from phosphine oxide-decorated polymer immobilized ionic liquid (O
PPh2-PIILS generated from phosphine oxide-decorated polymer immobilized ionic liquid (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh2-PIIL) impregnated with ruthenium trichloride is as efficient as that prepared directly from RuCl3 and PPh2-PIIL. Incorporation of PEG into the polymer support improved catalyst performance and the initial TOF of 2350 h−1 obtained for the aqueous phase hydrogenation of acetophenone is among the highest to be reported for a ruthenium nanoparticle-based catalyst. Under optimum conditions, RuNP@O
PPh2-PIIL) impregnated with ruthenium trichloride is as efficient as that prepared directly from RuCl3 and PPh2-PIIL. Incorporation of PEG into the polymer support improved catalyst performance and the initial TOF of 2350 h−1 obtained for the aqueous phase hydrogenation of acetophenone is among the highest to be reported for a ruthenium nanoparticle-based catalyst. Under optimum conditions, RuNP@O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh2-PEGPIILS recycled ten times with only a minor reduction in activity and no detectable change in selectivity. High yields and excellent selectivities were also obtained for hydrogenation of the C
PPh2-PEGPIILS recycled ten times with only a minor reduction in activity and no detectable change in selectivity. High yields and excellent selectivities were also obtained for hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O across a range of substituted aryl and heteroaryl ketones. Complete hydrogenation of the aromatic ring and C
O across a range of substituted aryl and heteroaryl ketones. Complete hydrogenation of the aromatic ring and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O could also be achieved by increasing the pressure and temperature accordingly. The same system also catalyzes the aqueous phase hydrogenation of furfural under mild conditions with an initial TOF of 3160 h−1 as well as the selective hydrogenation of levulinic acid and its ethyl ester to γ-valerolactone (GVL); reaction times for the latter could be reduced quite significantly by addition of either butyric acid or Amberlyst H-15.
O could also be achieved by increasing the pressure and temperature accordingly. The same system also catalyzes the aqueous phase hydrogenation of furfural under mild conditions with an initial TOF of 3160 h−1 as well as the selective hydrogenation of levulinic acid and its ethyl ester to γ-valerolactone (GVL); reaction times for the latter could be reduced quite significantly by addition of either butyric acid or Amberlyst H-15.


 Please wait while we load your content...
Please wait while we load your content...