Effects of high-temperature CeO2 calcination on the activity of Pt/CeO2 catalysts for oxidation of unburned hydrocarbon fuels
Abstract
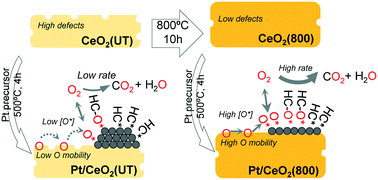
A CeO2-supported Pt catalyst (denoted Pt/CeO2(800)) was prepared by pre-calcining a commercial CeO2 support at high temperature (800 °C) before loading Pt via the incipient wetness impregnation method. Pt/CeO2(800) exhibits enhanced redox and hydrocarbon (HC) oxidation activity, in comparison to a catalyst with untreated CeO2 support (denoted Pt/CeO2(UT)). A combination of multiple characterization techniques, including X-ray diffraction (XRD), transmission electron microscopy (TEM), CO temperature-programed reduction (CO-TPR), and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), shows that the promoted redox activity of Pt/CeO2(800) is independent of the morphology of Pt clusters. Instead, it is associated with the enhanced mobility of surface lattice oxygen on the high temperature pretreated CeO2 support. The catalysts were evaluated for the catalytic oxidation of various HC fuel blendstocks with different functionalities (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and C
C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds) under different simplified and simulated exhaust conditions (with 0.74% and 10% O2). Under simulated exhaust conditions, fuel molecules, regardless of functionalities, are always more active under lean (high O2) conditions than under rich (low O2) conditions although their oxidation reactivity is suppressed by the competitive adsorption of NO and CO. The high redox activity of Pt/CeO2(800) facilitates the HC oxidation by accelerating the rate-limiting O2 activation step and broadening the operation window for an oxygen-dominant Pt surface.
O bonds) under different simplified and simulated exhaust conditions (with 0.74% and 10% O2). Under simulated exhaust conditions, fuel molecules, regardless of functionalities, are always more active under lean (high O2) conditions than under rich (low O2) conditions although their oxidation reactivity is suppressed by the competitive adsorption of NO and CO. The high redox activity of Pt/CeO2(800) facilitates the HC oxidation by accelerating the rate-limiting O2 activation step and broadening the operation window for an oxygen-dominant Pt surface.


 Please wait while we load your content...
Please wait while we load your content...