Facile preparation of MOF-derived MHCo3O4&Co/C with a hierarchical porous structure for entrapping enzymes: having both high stability and catalytic activity†
Abstract
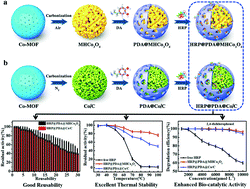
MOF-derived porous materials Co3O4 and Co/C with hierarchical structure (MHCo3O4&Co/C) were prepared by the self-sacrificial template strategy, and the surfaces of such materials were functionally modified with a “polydopamine (PDA)” bionic membrane for entrapping horseradish peroxidase (HRP) in this work. MHCo3O4 was prepared under air atmosphere, and Co/C was prepared under nitrogen atmosphere based on the thermolysis of Co-MOF. HRP@PDA@MHCo3O4 and HRP@PDA@Co/C could retain 96.8% and 93.7% catalytic activity compared to the free HRP, respectively, and exhibited better thermal stability and reusability. After 1 h incubation at 70 °C, HRP@PDA@MHCo3O4 and HRP@PDA@Co/C retained 84.8% and 70.8% of the initial activity compared with less than 20% retained by free HRP. After 10 cycles, HRP@PDA@MHCo3O4 and HRP@PDA@Co/C retained 92.4% and 85.4% of the initial activity. Moreover, HRP@PDA@MHCo3O4&Co/C exhibited extreme catalytic degradation efficiency for 2,4-dichlorophenol in simulated wastewater, and 2 mmol L−1 of 2,4-dichlorophenol was completely degraded within 15 min. The superior properties of HRP@PDA@MHCo3O4&Co/C were researched by kinetics and thermodynamics analyses. The results suggested that the mass transfer resistance of the substrates was lower than that of the bulk solution for the reason that the substrates were originally enriched on the hierarchical porous carrier close to the enzyme. The specificity and binding affinity of the enzymatic reactors to substrates were enhanced by the functionalized “PDA” bionic membrane on the carrier surface, compared with free enzyme in bulk buffer.


 Please wait while we load your content...
Please wait while we load your content...