Peptide mimotopes to emulate carbohydrates†
Abstract
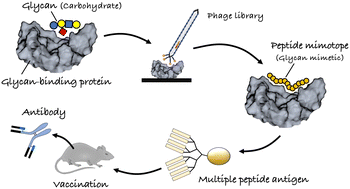
Glycoconjugates on animal cell surfaces are involved in numerous biological functions and diseases, especially the adhesion/metastasis of cancer cells, infection, and the onset of glycan-related diseases. In addition to glycoantigen detection, the regulation of glycan (carbohydrate)–protein interactions is needed to develop therapeutic strategies for glycan-related diseases. Preparation of a diverse range of glycan derivatives requires a massive effort, but the preparation and identification of alternative glycan-mimetic peptide mimotopes may provide a solution to this issue. Peptide mimotopes are recognized by glycan-binding proteins, such as lectins, enzymes, and antibodies, alternative to glycan ligands. Phage-display technology is the first choice in the selection of “glycan (carbohydrate)-mimetic peptide mimotopes” from a large repertoire of library sequences. This tutorial review describes the advantages of peptide mimotopes in comparison to glycan ligands, as well as their structural and functional mimicry. The detailed library design is followed by a description of the strategy used to improve affinity, and finally, an outline of the vaccine application of glycan-mimetic peptides is provided.
- This article is part of the themed collection: Exploring multivalent carbohydrate-protein interactions with chemical ligations


 Please wait while we load your content...
Please wait while we load your content...