Activation of aminocyclopropanes via radical intermediates
Abstract
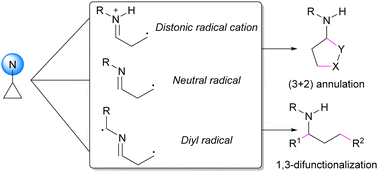
Aminocyclopropanes are versatile building blocks for accessing high value-added nitrogen-containing products. To control ring-opening promoted by ring strain, the Lewis acid activation of donor–acceptor substituted systems is now well established. Over the last decade, alternative approaches have emerged proceeding via the formation of radical intermediates, alleviating the need for double activation of the cyclopropanes. This tutorial review summarizes key concepts and recent progress in ring-opening transformations of aminocyclopropanes via radical intermediates, divided into formal cycloadditions and 1,3-difunctionalizations.


 Please wait while we load your content...
Please wait while we load your content...