Synthetic helical peptide capping strategies
Abstract
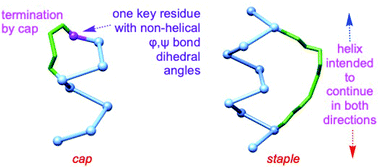
Relatively small mimics of interface secondary structures can be used to disrupt protein–protein interactions (PPIs). This strategy is valuable because many PPIs are pivotal in cell biology and contemporary medicinal chemistry. Small peptides tend to have random coil conformations in solution, so the entropy costs are high for them to order into states binding protein receptors. Consequently, peptides constrained in conformations resembling interface secondary structures are favored for enhanced affinities to PPI components. Helices are commonly found at PPI interfaces. The two general strategies that have emerged for imposing helical constraints in probes, capping and stapling, are often confused because both involve formation of macrocyclic rings. This review considers parameters that distinguish capping from stapling. Capping motifs terminate helices and project the adjacent peptide units in non-helical orientations, but stapling enforces helical motifs in ways that enable adjacent peptide fragments to extend helices. There is no evidence that stapling is more effective than capping for helix mimicry, but stapling is used more frequently. This imbalance may be because no strategies have emerged for synthetic C-capping with compact unit; if convenient and effective C-capping strategies were available then capping strategies should be more widely used.

 Please wait while we load your content...
Please wait while we load your content...