Ring contraction in synthesis of functionalized carbocycles
Abstract
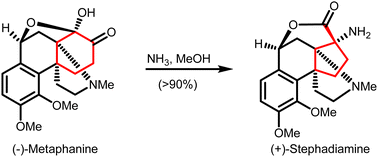
Carbocycles are a key and widely present structural motif in organic compounds. The construction of structurally intriguing carbocycles, such as highly-strained fused rings, spirocycles or highly-functionalized carbocycles with congested stereocenters, remains challenging in organic chemistry. Cyclopropanes, cyclobutanes and cyclopentanes within such carbocycles can be synthesized through ring contraction. These ring contractions involve re-arrangement of and/or small molecule extrusion from a parental ring, which is either a carbocycle or a heterocycle of larger size. This review provides an overview of synthetic methods for ring contractions to form cyclopropanes, cyclobutanes and cyclopentanes en route to structurally intriguing carbocycles.


 Please wait while we load your content...
Please wait while we load your content...