Beyond nylon 6: polyamides via ring opening polymerization of designer lactam monomers for biomedical applications
Abstract
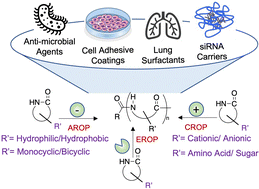
Ring opening polymerization (ROP) of lactams is a highly efficient and versatile method to synthesize polyamides. Within the last ten years, significant advances in polymerization methodology and monomer diversity are ushering in a new era of polyamide chemistry. We begin with a discussion of polymerization techniques including the most widely used anionic ring opening polymerization (AROP), and less prevalent cationic ROP and enzyme-catalyzed ROP. Next, we describe new monomers being explored for ROP with increased functionality and stereochemistry. We emphasize the relationships between composition, structure, and properties, and how chemists can control composition and structure to dictate a desired property or performance. Finally, we discuss biomedical applications of the synthesized polyamides, specifically as biomaterials and pharmaceuticals, with examples to include as antimicrobial agents, cell adhesion substrates, and drug delivery scaffolds.


 Please wait while we load your content...
Please wait while we load your content...