Beyond classical sulfone chemistry: metal- and photocatalytic approaches for C–S bond functionalization of sulfones
Abstract
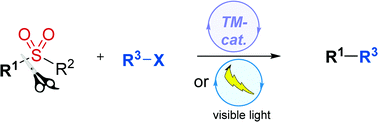
The exceptional versatility of sulfones has been extensively exploited in organic synthesis across several decades. Since the first demonstration in 2005 that sulfones can participate in Pd-catalysed Suzuki–Miyaura type reactions, tremendous advances in catalytic desulfitative functionalizations have opened a new area of research with burgeoning activity in recent years. This emerging field is displaying sulfone derivatives as a new class of substrates enabling catalytic C–C and C–X bond construction. In this review, we will discuss new facets of sulfone reactivity toward further expanding the flexibility of C–S bonds, with an emphasis on key mechanistic features. The inherent challenges confronting the development of these strategies will be presented, along with the potential application of this chemistry for the synthesis of natural products. Taken together, this knowledge should stimulate impactful improvements on the use of sulfones in catalytic desulfitative C–C and C–X bond formation. A main goal of this article is to bring this technology to the mainstream catalysis practice and to serve as inspiration for new perspectives in catalytic transformations.


 Please wait while we load your content...
Please wait while we load your content...