Formation of the acenaphthylene cation as a common C2H2-loss fragment in dissociative ionization of the PAH isomers anthracene and phenanthrene†
Abstract
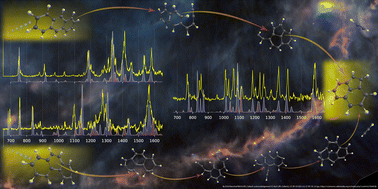
Polycyclic aromatic hydrocarbons (PAHs) are thought to be a major constituent of astrophysical environments, being the carriers of the ubiquitous aromatic infrared bands (AIBs) observed in the spectra of galactic and extra-galactic sources that are irradiated by ultraviolet (UV) photons. Small (2-cycles) PAHs were unambiguously detected in the TMC-1 dark cloud, showing that PAH growth pathways exist even at low temperatures. The processing of PAHs by UV photons also leads to their fragmentation, which has been recognized in recent years as an alternative route to the generally accepted bottom-up chemical pathways for the formation of complex hydrocarbons in UV-rich interstellar regions. Here we consider the C12H8+ ion that is formed in our experiments from the dissociative ionization of the anthracene and phenanthrene (C14H10) molecules. By employing the sensitive action spectroscopic scheme of infrared pre-dissociation (IRPD) in a cryogenic ion trap instrument coupled to the free-electron lasers at the FELIX Laboratory, we have recorded the broadband and narrow line-width gas-phase IR spectra of the fragment ions (C12H8+) and also the reference spectra of three low energy isomers of C12H8+. By comparing the experimental spectra to those obtained from quantum chemical calculations we have identified the dominant structure of the fragment ion formed in the dissociation process to be the acenaphthylene cation for both isomeric precursors. Ab initio molecular dynamics simulations are presented to elucidate the fragmentation process. This result reinforces the dominant role of species containing a pentagonal ring in the photochemistry of small PAHs.


 Please wait while we load your content...
Please wait while we load your content...