Reaction of lithium hexamethyldisilazide (LiHMDS) with water at ultracold conditions†
Abstract
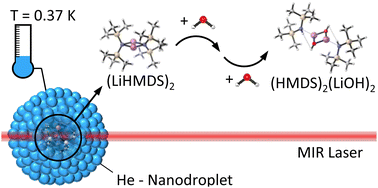
Alkali metal amides are highly reactive reagents that are broadly applied as strong bases in organic synthesis. Here, we use a combined helium nanodroplet IR spectroscopic and theoretical (DFT calculation) study to show that the reaction of the model compound lithium hexamethyldisilazide (LiHMDS) with water is close to barrierless even at ultra-cold conditions. Upon complex formation of dimeric (LiHMDS)2 with water in helium nanodroplets as ultra-cold nano-reactors (0.37 K) we observed the reaction product (LiOH)2(HMDS)2. This can be rationalized as aggregation induced reation upon stepwise addition of water. With increasing water partial pressure, only the product (LiOH)2(HMDS)2 is observed experimentally. This implies that the large interaction energy (69 kJ mol−1) of (LiHMDS)2 with water is sufficient to overcome the follow-up reaction barriers, in spite of the rapid cooling rates in He nanodroplets.
- This article is part of the themed collection: Festschrift Wolfgang E. Ernst: Electronic & Nuclear Dynamics in Molecules, Clusters, and on Surfaces


 Please wait while we load your content...
Please wait while we load your content...