Reliable experimental method for determination of photoacidity revealed by quantum chemical calculations†
Abstract
Photoacids are aromatic acids that exhibit significantly different acidities when they are electronically excited. Three experimental methods have been extensively used to determine the photoacidity, 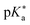 : fluorescence titration, the Förster cycle, and time-resolved experiments. However, the photoacidities determined by these experimental methods are not consistent. In this work, we used a theoretical method to evaluate the reliability of experimentally determined
: fluorescence titration, the Förster cycle, and time-resolved experiments. However, the photoacidities determined by these experimental methods are not consistent. In this work, we used a theoretical method to evaluate the reliability of experimentally determined 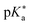 values. In particular, density functional theory (DFT) and time-dependent DFT calculations were used to obtain the changes in Gibbs free energy for acid dissociation reactions which are directly related to
values. In particular, density functional theory (DFT) and time-dependent DFT calculations were used to obtain the changes in Gibbs free energy for acid dissociation reactions which are directly related to 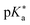 values. The Förster cycle, which is frequently used to experimentally determine the photoacidity due to its simplicity, yielded inconsistent results depending on how the transition energy was defined. We evaluated six empirical parameters extracted from the absorption and emission spectra of acidic and basic species of photoacids to adequately define the transition energy in the Förster cycle. And we found that the
values. The Förster cycle, which is frequently used to experimentally determine the photoacidity due to its simplicity, yielded inconsistent results depending on how the transition energy was defined. We evaluated six empirical parameters extracted from the absorption and emission spectra of acidic and basic species of photoacids to adequately define the transition energy in the Förster cycle. And we found that the 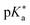 values obtained using the optical bandgap as the transition energy in the Förster cycle were in the best agreement with the results of quantum chemical calculations.
values obtained using the optical bandgap as the transition energy in the Förster cycle were in the best agreement with the results of quantum chemical calculations.

- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...