Iodine speciation in deep eutectic solvents†
Abstract
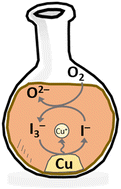
Iodine has been shown to act as a good electrocatalyst for metal digestion in deep eutectic solvents (DESs) but little is known about its speciation or reactivity in these high chloride containing media. Extended X-ray absorption fine structure (EXAFS) spectroscopy measurements were made at the iodine K-edge in a range of DESs with different glycolic or acidic hydrogen bond donors (HBDs), along with examining the effect of iodine concentration between 0.01 and 0.5 mol dm−3. Three groups of speciation were detected: mixed I2Cl−/I3− (glycol and lactic acid systems), mixed I3−/I2 (oxalic acid and urea systems), and singular I3− (levulinic acid system). UV-vis spectroscopy was used to confirm the speciation. Electrochemistry showed that iodine redox behaviour was unaffected by the changing speciation. Leaching data showed that metal oxidation was related not only to changing iodine speciation, but also the reactivity and coordination ability of the HBD.


 Please wait while we load your content...
Please wait while we load your content...