Surface-chemistry-driven water dissociation on cobalt-based graphene hybrid from molecular dynamics simulations†
Abstract
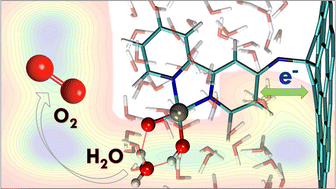
We explore the water dissociation process of graphene hybridized with a Co–bipyridine complex through first principles molecular dynamics simulations. We compute the free energies of the three main steps of the water dissociation process catalyzed by this hybrid catalyst. During the oxygen–oxygen bond formation, the transfer of the proton to the cis-OH of the cobalt complex is the rate determining step. The formation of the oxygen–oxygen bond leads to the peroxide complex, which is converted to the superoxide complex through the proton-coupled electron transfer (PCET) process. The formed superoxide complex is the rate-determining intermediate from which the oxygen molecule releases. The electronic properties of each state of the process were examined through Lowdin and Mulliken population analyses, local density of states (LDOS), and Wannier center analysis. The detailed electronic analysis reveals that the graphene sheet takes electronic density from the complex during the oxygen–oxygen bond formation. During the PCET process, the sheet shares the electron density with the complex, which stabilizes the superoxide complex. The Wannier center analysis provides evidence of the variation in the oxidation states of the metal center.


 Please wait while we load your content...
Please wait while we load your content...