Abstract
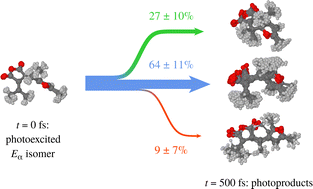
Furylfulgides, a class of photochromic organic compounds, show a complex system of photoinduced reactions. In the present study, the excited-state dynamics of the Eα and Eβ isomers of a representative furylfulgide is modelled with the use of nonadiabatic molecular dynamics simulations. Moreover, a pattern recognition algorithm is employed in order to automatically identify relaxation pathways, and to quantify the photoproduct distributions. The simulation results indicate that, despite differing only in the orientation of the furyl group, the two isomers show markedly different photochemical behaviour. The predominant Eα isomer undergoes photocyclisation with a quantum yield (QY) of 0.27 ± 0.10. For this isomer, the undesired E → Z photoisomerisation around the central double bond represents a minor side reaction, with a QY of 0.09 ± 0.07. In contrast, the minority Eβ isomer, which is incapable of photocyclisation, undergoes efficient E → Z photoisomerisation, with a QY as high as 0.56 ± 0.14. The relaxation kinetics and the photoproduct distributions are interpreted in the light of the available experimental data.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...