Pd speciation on black phosphorene in a CO and C2H4 atmosphere: a first-principles investigation
Abstract
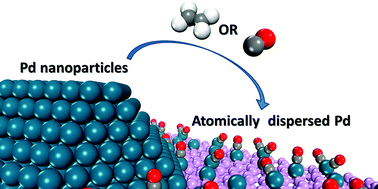
Deposited transition metal clusters and nanoparticles are widely used as catalysts and have long been thought stable in reaction conditions. We investigated the electronic structure and stability of freestanding and black phosphorene supported small Pd clusters containing 1 to 6 atoms in a CO or C2H4 atmosphere by extensive first-principles based calculations. We showed that, driven by the thermodynamics, subnanometric Pd clusters and single Pd atom on phosphorene may evolve for a better balance among metal–metal, metal–support and metal–adsorbate interactions, etc., resulting in atomic dispersion of Pd in reaction conditions. The strong interfacial Pd–P interactions would deform preformed Pd clusters into atomic strips of various lengths with enhanced stability comparable to bulk Pd. The diffusion barriers of terminal Pd atoms in the zigzag direction on phosphorene are small (<0.3 eV) and vary within ∼0.1 eV with the length of these atomic strips. Further adsorption of CO or C2H4 alters the Pd–P and Pd–Pd interactions and forms thermodynamically stable surface Pd species with decreased diffusion barriers, suggesting that atomic dispersion of Pd can be achieved on phosphorene, especially in a CO or C2H4 atmosphere. The current work may help to understand the superior catalytic performance of supported subnanometric transition metal catalysts in reaction conditions and pave the way for fabrication of single atom catalysts with the desired performance.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...