Nitric oxide electrochemical reduction reaction on transition metal-doped MoSi2N4 monolayers†
Abstract
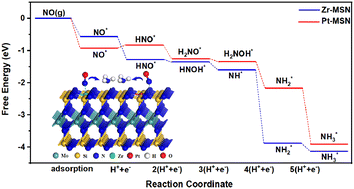
Nitric oxide electrochemical reduction (NOER) reactions are usually catalyzed by noble metals. However, the commercial applications are limited by the low atomic utilization and high price, which prompt researchers to turn their attentions to single-atom catalysts (SACs). Recently, a novel two-dimensional semiconducting material MoSi2N4 (MSN) has been synthesized and is suitable for the substrate of SACs due to its high stability, carrier mobility and mechanical strength. Herein, we employed first principles calculations to investigate the catalytic properties of transition metal doped MoSi2N4 monolayers (labelled as TM–MSN, where TM is a transition metal atom from 3d to 5d except Y, Tc, Cd, La–Lu and Hg) in NO reduction. The calculated results demonstrate that the introduction of Zr, Pd, Pt, Mn, Au, or Mo atoms can greatly improve the catalytic NOER performance of a pristine MSN monolayer. Zr–MSN and Pt–MSN monolayers at low coverage exhibit the most superior catalytic activity and selectivity for NH3 production with a limiting potential of 0 and −0.10 V. This work may help guide the application of MSN monolayer in the area of energy conversion.


 Please wait while we load your content...
Please wait while we load your content...