Insight into the inclusion of heteroatom impurities in silicon structures†
Abstract
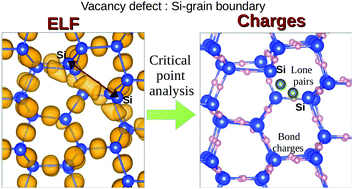
The bonding properties of the tilt boundary in poly-silicon and the effect of interstitial impurities are investigated by first-principles. In order to obtain thorough information on the nature of chemical bondings in these solid systems, an accurate topological analysis is performed, through partitioning of the electron localization function. Although the mechanism of segregation of single light impurities, such as carbon, nitrogen, and oxygen, in Si-based systems is known, it is only in the presence of multiple segregations that the distinctive structures of the various interstitial impurities emerge. The structural analysis of the modified Si systems and the comparison with the corresponding molecular structure within these solid phases provide an adequate description of interesting properties, for which bond charges provide more insight than bond length. It is shown that, in the presence of isovalent carbon, all systems try to preserve the tetrahedral coordination; on the contrary, trivalent nitrogen induces a strong local distortion to fit in the tetrahedral Si matrix while oxygen is the impurity that segregates more easily and more regularly. This work shows that impurities lead to local distortions and shows how the electron distribution rearranges to smooth these distortions. Overall, it shows how the analysis of bonds and their correlation with energetics and electronic structure is of fundamental importance for the understanding of the defects induced properties and of the basic mechanisms that influence them.


 Please wait while we load your content...
Please wait while we load your content...