Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates†
Abstract
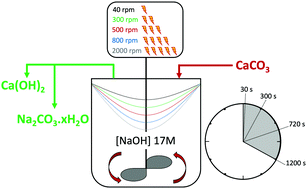
The decarbonisation of CaCO3 is essential for the production of lime (Ca(OH)2 and CaO), which is a commodity required in several large industries and the main precursor for cement production. CaCO3 is usually decarbonised at high temperatures, generating gaseous CO2 which will require post-process capture to minimise its release into the environment. We have developed a new process that can decarbonise CaCO3 under ambient conditions, while sequestering the CO2 as Na2CO3·H2O or Na2CO3 in the same stage. Here, the effects of increasing stirring rates and residence times on reaction efficiency of the key reaction occurring between CaCO3 and NaOH solution are studied. It is shown that the reaction is enhanced at lower stirring rates and longer residence times up to 300 seconds of contact between the reactants. The mass balance performed for Ca and CO2 revealed that up to the 95% of the process CO2 embodied in CaCO3 was sequestered, with maximum capture rate assessed at nn moles CO2 captured per second of reaction progress. A deeper insight into the precipitation of Na2CO3·H2O or Na2CO3 under different reaction conditions was gained, and SEM-EDX analysis enabled the observation of the reaction front by detection of Na migrating towards inner regions of partially-reacted limestone chalk particles.


 Please wait while we load your content...
Please wait while we load your content...