Hexachlorobenzene-negative ion formation in electron attachment experiments†
Abstract
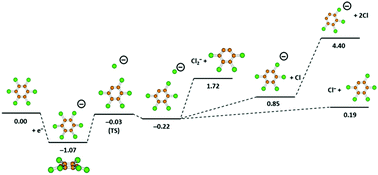
In this contribution, we report a comprehensive study on the formation of hexachlorobenzene (C6Cl6) negative ions probed by low-energy electron interactions from 0 up to 12 eV in a gas-phase crossed beam experiment. The anionic yields as a function of the electron energy reveal a rich fragmentation pattern of the dissociative electron attachment process, yet the most intense ion has been assigned to the non-dissociated parent anion that survives long enough within the detection time window. Other less intense fragment anions have been assigned as Cl−, Cl2−, C6Cl4−, and C6Cl5−. The experimental results are accompanied by quantum chemical calculations at various levels of accuracy, providing an insight into the electronic structure, thermochemical thresholds, electron affinities and structures of neutral and anionic molecular species. The electron attachment process induces a considerable geometry change in the temporary-negative ion relative to the neutral molecule, where the most intense fragment anion assigned to Cl− can be formed solely through a curve crossing involving a π*/σ* coupling. The yield of chlorine anions shows a signature of vibrational excitation reminiscent of a Jahn–Teller distortion.


 Please wait while we load your content...
Please wait while we load your content...