Singlet oxygen quenching as a probe for cytochrome c molten globule state formation†
Abstract
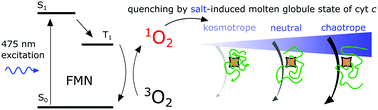
Singlet oxygen refers to the nonradical metastable excited state of molecular oxygen that readily oxidizes various cellular components. Its behavior in different biological systems has been studied for many years. Recently, we analyzed the effect of singlet oxygen quenching by heme cofactor in cytochrome c (cyt c). Here, we have exploited this effect in the investigation of conformational differences in the molten globule states of cyt c induced by different sodium anions, namely sulfate, chloride and perchlorate. The high efficiency of heme toward quenching singlet oxygen enabled us to use this property for the analysis of the otherwise experimentally difficult-to-determine parameter of heme upon exposure to solvents as highly similar conformational states of cyt c in the molten globule states are induced by different salts at acidic pH. Our results from singlet oxygen quenching experiments correlate well with other spectroscopic methods, such as circular dichroism and fluorescence measurements, and suggest increasing availability of heme in the order: perchlorate < chloride < sulfate. Based on our findings we propose that singlet oxygen phosphorescence measurements are useful in determining the differences in the protein conformation of their heme regions, particularly regarding the relative heme exposure to the solvent.


 Please wait while we load your content...
Please wait while we load your content...