Resonance-assisted intramolecular triel bonds†
Abstract
The possibility that the intramolecular Tr⋯S triel bond is strengthened by resonance is examined by quantum chemical calculations within the planar five-membered ring of TrH2–CR![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR–CR
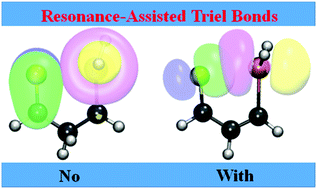
CR–CR![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (Tr = Al, Ga, In; R = NO2, CH3). This internal bond is found to be rather short (2.4–2.7 Å) with a large bond energy between 12 and 21 kcal mol−1. The pattern of bond length alternation and atomic charges within the ring is consistent with resonance involving the conjugated double bonds. This resonance enhances the triel bond strength by some 25%. The electron-withdrawing NO2 group weakens the bond, but it is strengthened by the electron-donating CH3 substituent. NICS analysis suggests the presence of a certain degree of aromaticity within the ring.
S (Tr = Al, Ga, In; R = NO2, CH3). This internal bond is found to be rather short (2.4–2.7 Å) with a large bond energy between 12 and 21 kcal mol−1. The pattern of bond length alternation and atomic charges within the ring is consistent with resonance involving the conjugated double bonds. This resonance enhances the triel bond strength by some 25%. The electron-withdrawing NO2 group weakens the bond, but it is strengthened by the electron-donating CH3 substituent. NICS analysis suggests the presence of a certain degree of aromaticity within the ring.


 Please wait while we load your content...
Please wait while we load your content...