Rearrangement and decomposition pathways of bare and hydrogenated molybdenum oxysulfides in the gas phase†
Abstract
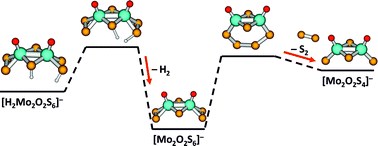
Molybdenum sulfides and molybdenum oxysulfides are considered a promising and cheap alternative to platinum as a catalyst for the hydrogen evolution reaction (HER). To better understand possible rearrangements during catalyst activation, we perform collision induced dissociation experiments in the gas phase with eight different molybdenum oxysulfides, namely [Mo2O2S6]2−, [Mo2O2S6]−, [Mo2O2S5]2−, [Mo2O2S5]−, [Mo2O2S4]−, [HMo2O2S6]−, [HMo2O2S5]− and [HMo2O2S4]−, on a Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer. We identify fragmentation channels of the molybdenum oxysulfides and their interconnections. Together with quantum chemical calculations, the results show that [Mo2O2S4]− is a particularly stable species against further dissociation, which is reached from all starting species with relatively low collision energies. Most interestingly, H atom loss is the only fragmentation channel observed for [HMo2O2S4]− at low collision energies, which relates to potential HER activity, since two such H atom binding sites on a surface may act together to release H2. The calculations reveal that multiple isomers are often very close in energy, especially for the hydrogenated species, i.e., atomic hydrogen can bind at various sites of the clusters. S2 groups play a decisive role in hydrogen adsorption. These are further features with potential relevance for HER catalysis.
- This article is part of the themed collection: Stability and properties of new-generation metal and metal-oxide clusters down to subnanometer scale


 Please wait while we load your content...
Please wait while we load your content...