Offsets between hyperconjugations, p→d donations and Pauli repulsions impact the bonding of E–O–E systems. Case study on elements of Group 14†
Abstract
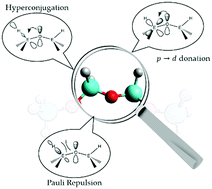
The nature of the E–O chemical bond (E = C, Si, Ge, Sn) is investigated in a wide range of model derivatives, such as oxonium cations, hydrogenated/methylated/fluorinated/chlorinated ethers and acyclic oligomers incorporating the E–O–E moiety. By means of density functional theory (DFT) calculations and natural bond orbital (NBO) techniques, we propose a bonding mechanism that explains the structural contrast between the organic and the inorganic counterparts of all these derivatives: the interplay between stabilizing interactions like LP(O)→σ*(E–X) hyperconjugations and LP(O)→d(E) donations with LP(O)⋯σ(E–X) vicinal Pauli repulsions (X = H, C, O, F, Cl) dictates the equilibrium structures in terms of E–O–E angles and E–O bond lengths. In addition, the present work represents the first study of oxonium ions that describes the structural discrepancies among organic derivatives and their heavier analogues. Another novel outcome for ethers and oligomers is that the two non-equivalent lone pair electrons (LPs) at the oxygen atoms impact in different manners the geometries of such derivatives, i.e. the s/p LP is correlated with the bending behaviour of the E–O–E units, while the pure p LP mainly dictates the short E–O bond distances of inorganic derivatives. Lastly, we evaluate the impact of the number of electronegative substituents, e.g. F, Cl or OEH3 groups, on the bond patterns developed for hydrogenated or methylated ethers.


 Please wait while we load your content...
Please wait while we load your content...