A theoretical study of asymmetric ketone hydrogenation catalyzed by Mn complexes: from the catalytic mechanism to the catalyst design†
Abstract
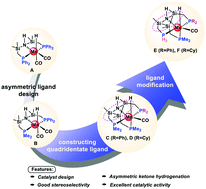
Herein, a density functional theory (DFT) study was performed to investigate asymmetric ketone hydrogenation (AKH) catalyzed by Mn complexes, from the catalytic mechanism to the catalyst design. The calculated results indicated that the Mn(CO)2–PSiNSiP (A1, PSiNSiP = P(Ph)2Si(CH3)2NSi(CH3)2P(Ph)2) pincer complex has potential high catalytic activity for ketone hydrogenation. The Mn(CO)–LYB (B, LYB = P(Ph)2Si(CH3)2NSi(CH3)2P(Me)2) pincer complex was then designed to catalyze AKH with good stereoselectivity. The hydrogen transfer (HT) step is the chirality-determining step. To avoid the enantiomer of Mn(CO)2–LYB, which could eliminate the high stereoselectivity during AKH, novel Mn complexes with quadridentate ligands, such as Mn(CO)–LYC (C, LYC = P(CH3)2CH2Si(CH3)NSi(CH3)(Si(CH3)CH2P(CH3)2)CH2P(Ph)2) and Mn(CO)–LYD (D, LYD = P(CH3)2CH2Si(CH3)NSi(CH3)(Si(CH3)CH2P(CH3)2)CH2P(Cy)2), were designed to drive AKH with medium stereoselectivity. In order to increase the stereoselectivity of AKH, Mn(CO)–LYE (E, LYE = PH2CH2Si(CH3)NSi(CH3)(Si(CH3)CH2P(CH3)2)CH2P(Ph)2) and Mn(CO)–LYF (F, LYF = PH2CH2Si(CH3)NSi(CH3)(Si(CH3)CH2P(CH3)2)CH2P(Cy)2) were further designed and showed very good stereoselectivity, which is due to the lower deformation energy and stronger interactions between the ketone substrates and catalysts. This work may shed light on the design of cheap metal catalysts with a new ligand framework for the asymmetric hydrogenation (AH) of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bonds (X = O, N).
X bonds (X = O, N).


 Please wait while we load your content...
Please wait while we load your content...