Single atom alloys vs. phase separated alloys in Cu, Ag, and Au atoms with Ni(111) and Ni, Pd, and Pt atoms with Cu(111): a theoretical exploration†
Abstract
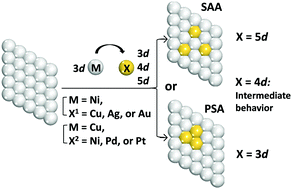
A single-atom alloy (SAA) consisting of an abundant metal host and a precious metal guest is a promising catalyst to reduce the cost without a loss of activity. DFT calculations of Ni- and Cu-based alloys nX/M(111) (X = Cu, Ag, or Au for M = Ni; X = Ni, Pd, or Pt for M = Cu; n = 1–4) reveal that a phase-separated alloy (PSA) is produced by Cu atoms with Ni(111) but an SAA is produced by Au atoms with Ni(111) and Pd and Pt atoms with Cu(111). In the Ni(111)-based Ag alloy and Cu(111)-based Ni alloy, the relative stabilities of the SAA and PSA depend on coverages of Ag on Ni(111) and Ni on Cu(111). The interaction energy (Eint) between the Xn cluster and M(111) host is larger than that between one X atom and the M(111) host, because the Xn cluster forms more bonding interactions with the M(111) host than does one X atom. When going from one X atom to the X4 cluster, the Eint values of Au and Pt clusters respectively with Ni(111) and Cu(111) increase to a lesser extent than those of Cu and Ni clusters respectively with Ni(111) and Cu(111). Consequently, Au and Pt atoms tend to form SAAs respectively with Ni(111) and Cu(111) hosts compared to Cu and Ni atoms. This trend in the Eint value is determined by the valence orbital energies of the X atom and the Xn cluster. Cu atoms in nCu/Ni(111) have a slightly positive charge but Ag atoms in nAg/Ni(111), Au atoms in nAu/Ni(111), and Ni, Pd, and Pt atoms in nX/Cu(111) (X = Ni, Pd, or Pt) have a negative charge. The negative charge increases in the order Ag < Au in nX/Ni(111) and Ni < Pd < Pt in nX/Cu(111). The Fermi level decreases in energy in the order nCu/Ni(111) ≥ Ni(111) > nAg/Ni(111) > nAu/Ni(111) and Cu(111) ≥ nNi/Cu(111) > nPd/Cu(111) > nPt/Cu(111). The d valence band center decreases in energy in almost the same order. The CO adsorption energy decreases in the order Ni(111) ∼ nCu/Ni(111) > nAg/Ni(111) ∼ nAu/Ni(111) and Cu(111) > nNi/Cu(111) > nPd/Cu(111) > nPt/Cu(111). These properties are explained based on the electronic structures.


 Please wait while we load your content...
Please wait while we load your content...