Ultrafast proton transfer of the aqueous phenol radical cation†
Abstract
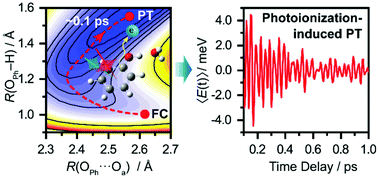
Proton transfer (PT) reactions are fundamental to numerous chemical and biological processes. While sub-picosecond PT involving electronically excited states has been extensively studied, little is known about ultrafast PT triggered by photoionization. Here, we employ femtosecond optical pump-probe spectroscopy and quantum dynamics calculations to investigate the ultrafast proton transfer dynamics of the aqueous phenol radical cation (PhOH˙+). Analysis of the vibrational wave packet dynamics reveals unusually short dephasing times of 0.18 ± 0.02 ps and 0.16 ± 0.02 ps for the PhOH˙+ O–H wag and bend frequencies, respectively, suggestive of ultrafast PT occurring on the ∼0.1 ps timescale. The reduced potential energy surface obtained from ab initio calculations shows that PT is barrierless when it is coupled to the intermolecular hindered translation between PhOH˙+ and the proton-acceptor water molecule. Quantum dynamics calculations yield a lifetime of 193 fs for PhOH˙+, in good agreement with the experimental results and consistent with the PT reaction being mediated by the intermolecular O⋯O stretch. These results suggest that photoionization can be harnessed to produce photoacids that undergo ultrafast PT. In addition, they also show that PT can serve as an ultrafast deactivation channel for limiting the oxidative damage potential of radical cations.
- This article is part of the themed collections: Festschrift Wolfgang E. Ernst: Electronic & Nuclear Dynamics in Molecules, Clusters, and on Surfaces and 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...