Iodous acid – a more efficient nucleation precursor than iodic acid†
Abstract
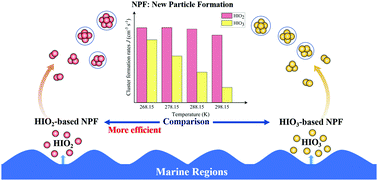
Iodous acid (HIO2), a vital iodine oxyacid, potentially plays an important role in the formation of new particles in marine areas (He et al., Science, 2021, 371, 589–595). However, the nucleation mechanism of HIO2 is still poorly understood. Herein, the self-nucleation of HIO2 under different atmospheric conditions is investigated by a combination of quantum chemical calculations and the Atmospheric Cluster Dynamics Code (ACDC) simulations. The results indicate that HIO2 can form relatively stable molecular clusters through hydrogen bonds and halogen bonds, and the self-nucleation of HIO2 proceeds by sequential addition of HIO2 or HIO2-based small clusters. Besides, in order to better illustrate the role of HIO2 in new particle formation (NPF) in marine areas, we compare its nucleation properties with those of iodic acid (HIO3), a significant iodine-containing nucleation precursor in marine regions. We find that the cluster formation rate of the self-nucleation of HIO2 is higher than that of the self-nucleation of HIO3 although [HIO2] is lower than [HIO3], which indicates that the HIO2 molecule is a more efficient nucleation precursor than the HIO3 molecule. Therefore, the self-nucleation of HIO2 could become one of the most important sources for NPF in marine areas, which could provide potential theoretical evidence for explaining the intensive NPF events observed in these areas.


 Please wait while we load your content...
Please wait while we load your content...