Photoexcitation dynamics of bromodiphenyl ethers in acetonitrile-d3 studied by femtosecond time-resolved infrared spectroscopy†
Abstract
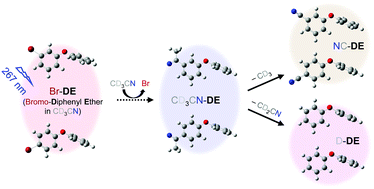
The efficient decomposition of polybrominated diphenyl ethers (PBDEs), onetime prevalent flame retardants, is central to the reduction of their harmful effects on human health. PBDE photodecomposition is a promising method, but its mechanism and products are not well understood. The photoexcitation dynamics of 3- and 4-bromodiphenyl ethers (BDE-2 and BDE-3) in CD3CN were studied from 0.3 ps to 10 μs using time-resolved infrared spectroscopy. An excitation at 267 nm dissociated the Br atom from BDE-2 and BDE-3 within 0.3 ps and 14 ± 3 ps, respectively, producing a radical compound (R) and a Br atom. About 85% of R formed an intermediate (IM) that weakly interacted with the Br atom and the surrounding CD3CN solvent in 7–12 ps. The remaining R separated from the dissociated Br and underwent slow geminate rebinding (GR) with Br within 35 to 54 ns. The IM competitively engaged in GR with the interacting Br in 40–60 ps or formed CD3CN-bound radical compounds (RS) in 100–130 ps. The RS further degraded via either the dissociation of CD3—producing a cyano-bound diphenyl ether (DE) in 150 or 550 ns—or the deuterium abstraction of CD3CN in 180 or 430 ns—producing a deuterated DE. Overall, 33 ± 3 (22 ± 3)% of the photoexcited BDE-2 (BDE-3) decomposed in CD3CN under 267 nm excitation. Efficient binding of the CD3CN solvent to R deterred the yield-diminishing GR and slowed the rate of product formation. The observed photoexcitation dynamics of BDE suggest methods for the efficient decomposition of PBDE.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...