Theoretical study of the NO3 radical reaction with CH2ClBr, CH2ICl, CH2BrI, CHCl2Br, and CHClBr2†
Abstract
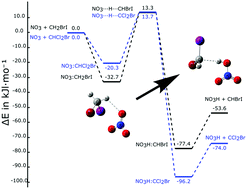
The potential reaction of the nitrate radical (NO3), the main nighttime atmospheric oxidant, with five alkyl halides, halons (CH2ClBr, CH2ICl, CH2BrI, CHCl2Br, and CHClBr2) has been studied theoretically. The most favorable reaction corresponds to a hydrogen atom transfer. The stationary points on the potential energy surfaces of these reactions have been characterized. The reactions can be classified into two groups based on the number of hydrogen atoms in the halon molecules (1 or 2). The reactions with halons with only one hydrogen atom show more exothermic profiles than those with two hydrogen atoms. In addition, the kinetics of the reaction of NO3 + CH2BrI was studied in much higher detail using a multi-well Master Equation solver as a representative example of the nitrate radical reactivity against these halocarbons. These results indicate that the chemical lifetime of the alkyl halides would not be substantially affected by nitrate radical reactions, even in the case of NO3-polluted atmospheric conditions.
- This article is part of the themed collection: Celebrating our 2025 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...