Examination of the Brønsted–Evans–Polanyi relationship for the hydrogen evolution reaction on transition metals based on constant electrode potential density functional theory†
Abstract
In the search for efficient and inexpensive electrocatalysts for the hydrogen evolution reaction (HER), the hydrogen binding energy 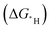 is often used as a descriptor to represent the catalytic activity. The success of this approach relies on the Brønsted–Evans–Polanyi (BEP) relationship. In this study, we used constant electrode potential density functional theory calculations to examine this relationship. Eight fcc metals with a low hydrogen adsorption concentration of 1/9 were used as the model systems. We found that the HER kinetic barriers are indeed correlated to the
is often used as a descriptor to represent the catalytic activity. The success of this approach relies on the Brønsted–Evans–Polanyi (BEP) relationship. In this study, we used constant electrode potential density functional theory calculations to examine this relationship. Eight fcc metals with a low hydrogen adsorption concentration of 1/9 were used as the model systems. We found that the HER kinetic barriers are indeed correlated to the 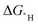 . Both the
. Both the 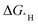 s of the hollow site and less favourable top site correlate to the kinetic barriers; however, the correlation is better for the latter. This behaviour leads to a set of equations for estimating the HER kinetic barriers with improved accuracy that can be used to predict the HER performance of the materials with a low hydrogen adsorption concentration. This work demonstrates the importance of calculating the
s of the hollow site and less favourable top site correlate to the kinetic barriers; however, the correlation is better for the latter. This behaviour leads to a set of equations for estimating the HER kinetic barriers with improved accuracy that can be used to predict the HER performance of the materials with a low hydrogen adsorption concentration. This work demonstrates the importance of calculating the 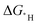 of a suitable adsorption site to establish good BEP relationships.
of a suitable adsorption site to establish good BEP relationships.



 Please wait while we load your content...
Please wait while we load your content...