Pressure-induced evolution of crystal and electronic structure of neptunium hydrides†
Abstract
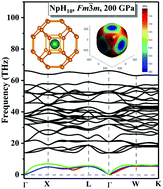
An extensive exploration of high-pressure phase diagrams of NpHx (x = 1–10) compounds was performed by using swarm-intelligence-based CALYPSO structure searches. We propose five stable hydrogen-rich clathrate phases (P4/nmm-NpH5, Cmcm-NpH7, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-NpH8, P63/mmc-NpH9, and Fm
m-NpH8, P63/mmc-NpH9, and Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-NpH10) that are composed of unusual H cages with stoichiometries H20, H24, H29, and H32 in which the H atoms are weakly covalently bonded to one another, with neptunium atoms occupying centers of the cages. The electronic structure analyses show that these predicted hydrogen-rich structures are all metallic phases, and Np–H and H–H bonds are formed by ionic and covalent bond interactions, respectively. The charge transfer from the Np atom plays an important role in the stability of the proposed structures. All hydrogen-rich clathrate structures show superconductivity behavior in their respective stability pressure range. Our work is an important step in understanding the phase stability and bonding behavior of NpHx under extreme conditions and provides a valuable reference for experimental synthesis and identification of cage-like neptunium hydrides.
m-NpH10) that are composed of unusual H cages with stoichiometries H20, H24, H29, and H32 in which the H atoms are weakly covalently bonded to one another, with neptunium atoms occupying centers of the cages. The electronic structure analyses show that these predicted hydrogen-rich structures are all metallic phases, and Np–H and H–H bonds are formed by ionic and covalent bond interactions, respectively. The charge transfer from the Np atom plays an important role in the stability of the proposed structures. All hydrogen-rich clathrate structures show superconductivity behavior in their respective stability pressure range. Our work is an important step in understanding the phase stability and bonding behavior of NpHx under extreme conditions and provides a valuable reference for experimental synthesis and identification of cage-like neptunium hydrides.


 Please wait while we load your content...
Please wait while we load your content...