Influence of N-introduction in pentacene on the electronic structure and excited electronic states
Abstract
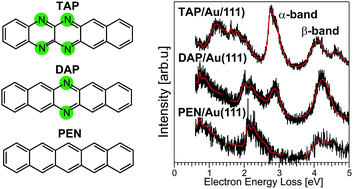
N-Heteropolycyclic aromatic compounds are promising organic semiconductors for applications in field effect transistors and solar cells. Thereby the electronic structure of organic/metal interfaces and thin films is essential for the performance of organic-molecule-based devices. Here, we studied the structural and the electronic properties of 6,7,12,13-tetraazapentacene (TAP) adsorbed on Au(111) using vibrational and electronic high-resolution electron energy loss spectroscopy in combination with state-of-the-art quantum chemical calculations. In the mono- and multilayer TAP adsorbs in a planar adsorption geometry with the molecular backbone oriented parallel to the gold substrate. The energies of the lowest excited electronic singlet states (S) as well as the triplet state (T) are assigned. The optical gap (S0 → S1 transition) is found to be 1.6 eV and the T1 energy 1.2 eV. In addition, thorough comparison to previously studied pentacene (PEN) and 6,13-diazapentacene (6,13-DAP) is made explaining in detail the influence of nitrogen substitution on the electronic structure and in particular on the intensity of the α-band in the UV/vis absorption spectrum. In the series PEN, 6,13-DAP, and TAP, the α-band (S0 → S2 transition) gains significantly in intensity due to individual effects of the introduced nitrogen atoms on the orbital energies.


 Please wait while we load your content...
Please wait while we load your content...