Synergetic ligand and size effects of boron cage based electrolytes in Li-ion batteries
Abstract
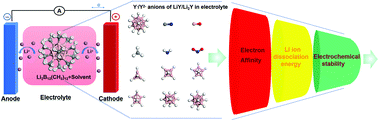
We explore the potential application of boron-based clusters as high-performance electrolytes in lithium-ion batteries using first-principles density functional theory. We use small and halogen-free ligands (such as CN, BO, NH2, NO2, and CH3) to replace H in closo-boron cages with different sizes to investigate the ligand and size effects. According to their geometric and electronic stability, Li+ dissociation energy in the lithium salt form, and electrochemical stabilities, we screen nine candidate electrolyte anions potentially overcoming the currently used electrolytes in lithium-ion batteries. We show that, when CH3 is used as a boron cage ligand, both the highest occupied molecular orbital and the lowest unoccupied molecular orbital levels are high, ensuring their electrochemical stability against the oxidation (or reduction) reaction at the anode (or cathode). Solvent effects are also evaluated and high electrostatic screening was found to be favorable for practical usage.


 Please wait while we load your content...
Please wait while we load your content...