Macromolecular crowding: how shape and interaction affect the structure, function, conformational dynamics and relative domain movement of a multi-domain protein†
Abstract
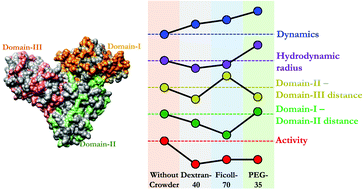
The cellular environment is crowded by macromolecules of various sizes, shapes, and charges, which modulate protein structure, function and dynamics. Herein, we contemplated the effect of three different macromolecular crowders: dextran-40, Ficoll-70 and PEG-35 on the structure, active-site conformational dynamics, function and relative domain movement of multi-domain human serum albumin (HSA). All the crowders used in this study have zero charges and similar sizes (at least in the dilute region) but different shapes and compositions. Some observations follow the traditional crowding theory. For example, all the crowders increased the α-helicity of HSA and hindered the conformational fluctuation dynamics. However, some observations are not in line with the expectations, such as an increase in the size of HSA with PEG-35 and uncorrelated domain movement of HSA with Ficoll-70 and PEG-35. The relative domain movement is correlated with the activity, suggesting that such moves are essential for protein function. The interaction between HSA and Ficoll-70 is proposed to be hydrophobic in nature. Overall, our results provide a somewhat systematic study of the shape-dependent macromolecular crowding effect on various protein properties and present a possible new insight into the mechanism of macromolecular crowding.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...