Electronic structures of hydroxylated low index surfaces of rutile and anatase-type titanium dioxide†
Abstract
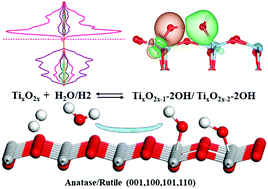
Different surface planes of various types of titanium dioxide (TiO2) crystals have diverse catalysis effects on the splitting of H2O and H2 and the electronic structures of the formed hydroxylated TiO2 vary significantly. A series of sixteen types of hydroxylated TiO2 surfaces containing two types of hydroxyls (OH1 and OH2) on four kinds of low index surfaces [(001), (100), (101), and (110)] of two types of crystals [anatase (A) and rutile (R)] are studied using first-principles density functional theory calculations. The catalyzed splitting of H2O and H2 on the eight low index surfaces is compared using Gibbs free energy. The geometries and electronic structures including the total and partial density of states and the charge density distribution of the sixteen hydroxylated surfaces are systematically described. The electronic structures of R-101, R-001, A-110, A-100, and A-001 surfaces are more significantly influenced by hydroxylation than other surfaces and the effects of OH2 are larger than those of OH1. In particular, the band gap values decrease and a new electronic energy state appears in R-001-OH2 and A-100-OH2. A new electronic state appears in the middle of the bands of R-101 and A-110 surfaces upon hydroxylation. The electron spin balance at the edge of the conduction band minimum of A-001-OH2 is disturbed. This research can provide theoretical guidance for experimental researchers to design surface hydroxylated TiO2 materials with tunable electronic structures and high catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...