How many methanol molecules effectively solvate an excess proton in the gas phase? Infrared spectroscopy of H+(methanol)n–benzene clusters†
Abstract
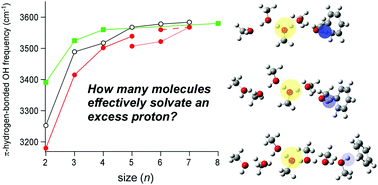
An excess proton in a hydrogen-bonded system enhances the strength of hydrogen bonds of the surrounding molecules. The extent of this influence can be a measure of the number of molecules effectively solvating the excess proton. Such extent in methanol has been discussed by the observation of the π-hydrogen-bonded OH stretch bands of the terminal sites of protonated methanol clusters, H+(methanol)n, in benzene solutions, and it has been concluded that ∼8 molecules effectively solvate the excess proton (Stoyanov et al., Chem. Eur. J. 2008, 14, 3596–3604). In the present study, we performed infrared spectroscopy of H+(methanol)n–benzene clusters in the gas phase. The cluster size and hydrogen-bonded network structure are identified by the tandem mass spectrometric technique and the comparison of the observed infrared spectra with density functional theory calculations. Though changes of the preferred hydrogen bond network type occur with the increase of cluster size in the gas phase clusters, the observed size dependence of the π-hydrogen bonded OH frequency agrees well with that in the benzene solutions. This means that the observations in both the gas and condensed phases catch the same physical essence of the excess proton solvation by methanol.


 Please wait while we load your content...
Please wait while we load your content...