Formation of protonated water–hydrogen clusters in an ion trap mass spectrometer at room temperature†
Abstract
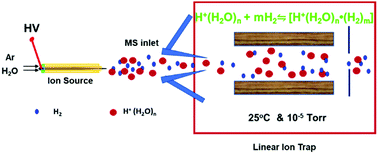
Protonated water–hydrogen clusters [H+(H2O)n·m(H2)] present an interesting model for fundamental water research, but their formation and isolation presents considerable experimental challenges. Here, we report the detection of [H+(H2O)n·m(H2)] (2 ≤ n ≤ 3, m ≤ 2) clusters alongside protonated water clusters H+(H2O)n (2 ≤ n ≤ 3) in a linear ion trap mass spectrometer under two different experimental conditions: (1) when water vapor was ionized by +5.5 kV ambient corona discharge in front of the mass spectrometer inlet; (2) when isolated H+(H2O)n clusters were exposed to H2 gas inside the linear trap. Chemical assignment of [H+(H2O)n·m(H2)] clusters was confirmed using reference experiments with isotopically labeled water and deuterium. Also, the formation of H2 gas in the corona discharge area was indicated by a flame test. Overall, our findings clearly indicate that [H+(H2O)n·m(H2)] clusters can be produced at room temperature through the association of protonated water clusters H+(H2O)n with H2 gas, without any cooling necessary. A mechanism for the formation of the protonated water–hydrogen complexes was proposed. Our results also suggest that the association of water ions with H2 gas may play a notable role in corona discharge ionization processes, such as atmospheric pressure chemical ionization, and may be partially responsible for the stabilization of reactive radical species occasionally reported in corona discharge ionization experiments.


 Please wait while we load your content...
Please wait while we load your content...