Anionic states of C6Cl6 probed in electron transfer experiments†
Abstract
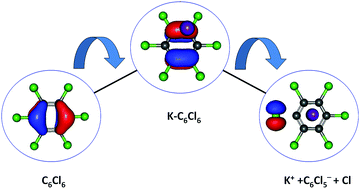
This is the first comprehensive investigation on the anionic species formed during collisions of fast neutral potassium (K) atoms with neutral hexachlorobenzene (C6Cl6) molecules in the laboratory frame range from 10 up to 100 eV. In such ion-pair formation experiments we also report a novel K+ energy loss spectrum obtained in the forward scattering giving evidence of the most accessible electronic states. The vertical electron affinity of (−3.76 ± 0.20) eV has been obtained and assigned to a purely repulsive transition from the C6Cl6 ground state to a 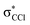 state of the temporary negative ion yielding Cl− formation. These experimental findings are also supported by state-of-the art theoretical calculations on the electronic structure of C6Cl6 in the presence of a potassium atom and are used for analysing the lowest unoccupied molecular orbitals participating in the collision process. From the time-of-flight mass spectra recorded in the wide collision energy range, more than 80% of the total anion yield is due to the undissociated parent anion C6Cl6−, C6Cl5− and Cl− formation. Other fragment anions such as C6Cl4−, C3Cl2−, C2Cl− and Cl2− that undergo complex internal reactions with the temporary negative ion formed after electron transfer account for less than 20% of the total yield. The joint experimental and theoretical methodologies employed in these electron transfer studies provide the most comprehensive and unique assignments of the hexachlorobenzene anionic species and the role of C6Cl6 electronic states in collision induced dissociation to date.
state of the temporary negative ion yielding Cl− formation. These experimental findings are also supported by state-of-the art theoretical calculations on the electronic structure of C6Cl6 in the presence of a potassium atom and are used for analysing the lowest unoccupied molecular orbitals participating in the collision process. From the time-of-flight mass spectra recorded in the wide collision energy range, more than 80% of the total anion yield is due to the undissociated parent anion C6Cl6−, C6Cl5− and Cl− formation. Other fragment anions such as C6Cl4−, C3Cl2−, C2Cl− and Cl2− that undergo complex internal reactions with the temporary negative ion formed after electron transfer account for less than 20% of the total yield. The joint experimental and theoretical methodologies employed in these electron transfer studies provide the most comprehensive and unique assignments of the hexachlorobenzene anionic species and the role of C6Cl6 electronic states in collision induced dissociation to date.


 Please wait while we load your content...
Please wait while we load your content...