Construction of bimetallic FeCo–SA/DABCO nanosheets by modulating the electronic structure for improved electrocatalytic oxygen evolution†
Abstract
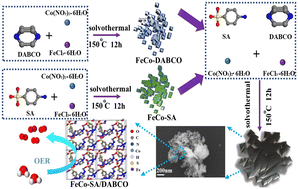
For energy conversion and storage, the electrochemical oxygen evolution process (OER) is the crucial half-reaction process. The amphiprotic characteristics of sulfamic acid are due to its amine and sulphonic acid functional groups. Metal-based catalysts can be utilized to fabricate bimetallic and dual-ligand linked FeCo–SA/DABCO nanosheets with improved catalytic performance. The crystal structure of bimetallic FeCo–SA/DABCO consisted of alternating organic hydrocarbons SA and DABCO and inorganic metal–oxygen layers. FeCo–SA/DABCO provided an overpotential (η10) of 290 mV to achieve a current density of 10 mA cm−2 together with a small Tafel slope of 112.79 mV dec−1 and also exhibited long-term durability. Electrochemical results showed that FeCo–SA/DABCO was superior to FeCo–SA and Co–SA/DABCO. Density functional theory calculations indicated that the theoretical overpotential of FeCo–SA/DABCO is 0.62 eV, which was lower than that of FeCo–SA. The introduction of composite SA/DABCO ligands can change the rate-determining step, which can improve the conductivity and reduce the reaction barrier for the formation of intermediates, thereby affecting the catalytic activity and changing the overall electron transfer capacity.


 Please wait while we load your content...
Please wait while we load your content...