Co-crystallization studies of the syn- and anti-atropisomers of triphenyl-based perfluorinated halogen bond donors with halides†
Abstract
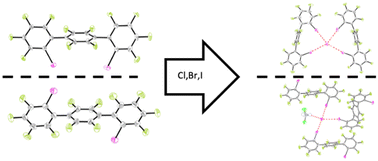
After recent two-point recognition studies using polyfluorinated twofold-iodinated 1,3- and 1,4-substituted triphenyl-based halogen bond (XB) donors, further crystallographic studies towards their binding behaviour were performed. To this end, syn- and anti-isomers of the XB donors were crystallized as pure compounds, as complexes with the halides chloride, bromide and iodide, and were investigated using X-ray diffraction. All complexes obtained showed strong halogen bonds with varying geometry and stoichiometry. The acquired data was then also compared to that of previously investigated fourfold-iodinated XB donors. This comparison showed strong differences again in geometry and stoichiometry, as well as slight differences in XB strength.


 Please wait while we load your content...
Please wait while we load your content...