Lanthanide(iii)-oxamato complexes containing Nd3+ and Ho3+: crystal structures, magnetic properties, and ab initio calculations†
Abstract
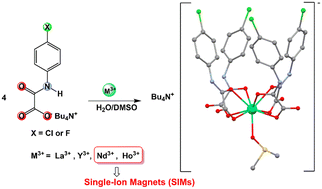
In this work, we present eight oxamato complexes of formula (n-Bu4N)[M(HL)4(DMSO)]·nH2O (M = Y3+, La3+, Nd3+, Ho3+) with either _HClppa (= N-(4-chlorophenyl)oxamic acid) or _HFppba (= N-(4-fluorophenyl)oxamic acid) as the HL ligands, n = 2 (_HFppa) or 3 (_HClppa), besides the coordinated dimethylsulfoxide (DMSO) molecule, and tetrabutylammonium (n-Bu4N+) as counterions. All compounds were characterized by elemental analysis, IR spectroscopy, and single-crystal X-ray diffraction. The analysis shows that all mononuclear compounds, with two oxygen atoms from each oxamic acid ligand and a DMSO molecule coordinated to the metal ion, result in a MO9 sphere of coordination. The dc magnetic properties of complexes Nd_HClppa, Nd_HFppa, Ho_HClppa, and Ho_HFppa reveal their paramagnetism. The ac magnetic measurements indicate the existence of slow relaxation of the magnetization for Ho3+ and Nd3+ compounds at low temperatures, suggesting that Ho3+ complexes are single-ion magnets (SIMs) while Nd3+ compounds are field-induced SIMs. Ab initio calculations were performed to determine the crystal-field parameters, which were subsequently used to obtain the best-fit curves of dc magnetic data and simulate susceptibility curves. The latter was consistent with experimental results for each compound, although small differences between compounds with the same lanthanide were not reproduced when all compounds were considered.


 Please wait while we load your content...
Please wait while we load your content...