Structural CO2 capture preference of semiclathrate hydrate formed with tetra-n-butylammonium chloride†
Abstract
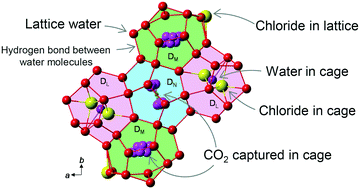
The crystal structure of tetra-n-butylammonium chloride (TBAC) + CO2 semiclathrate hydrate that is known to have high CO2 selectivity was studied by single crystal X-ray diffraction. The presence of the CO2 gas leads to occupation of dodecahedral (D) cages by water, CO2 and chloride. This hydrate structure has three types of D cages, i.e., DL, DM and DN, with the ratio for cage number DL : DM : DN = 2 : 2 : 1. CO2 occupied DM and DN cages which were suitably distorted for the linear CO2 molecular shape. Chloride anions partly occupied the DM cages, coordinating to water molecules. The lateral distortion of the DM and DN cage leads restricted spatial CO2 distribution in the cages. The DL cages were highly distorted by the TBA cation. In DL cages, partial occupancies with both water and chloride, which coordinated to cage water molecules, were found. Contrary to the water molecules which were placed at the cage center, chloride anions were off-centred at two distinct positions due to slightly longer coordination lengths with the cage water molecules. The present analysis showed a superior CO2 capture preference of TBAC hydrate in the structural aspect.


 Please wait while we load your content...
Please wait while we load your content...