Effect of chelator content on the structural and electrochemical performance of Na3V2(PO4)2F3 by sol–gel preparation†
Abstract
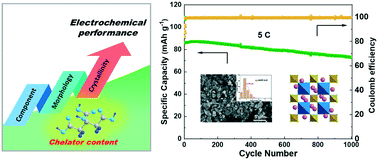
Na3V2(PO4)2F3 (NVPF) has been regarded as one of the most promising commercial cathode materials for sodium-ion batteries due to its high operating voltage and fast sodium-ion diffusion rates. The facile sol–gel method is generally conducted to ameliorate its low electronic conductivity by forming nanoparticles with a homogenous carbon coating. However, the effect of chelator content on the morphology and crystal structure of NVPF has been rarely studied. Herein, we conducted a comprehensive investigation of NVPF synthesized by sol–gel methods with various citric acid contents. The citric acid content severely affects the valence state of the V element, the bonding state of the F element, and the carbon content, particle size, crystal structure, and crystallinity of NVPF samples. More importantly, we found that the specific capacity, rate, and cycle performances are heavily dependent on NVPF crystallinity. With an optimized citric acid content, the NVPF-0.67 sample exhibits high specific capacities of 112.9 and 88.9 mAh g−1 at 0.2 and 10 C-rate, respectively, and good cycle stability with an 84.6% capacity retention after 1000 cycles at 5 C-rate. These results reveal the complicated relationship between the chelator content, morphology, crystal structure, and electrochemical properties, providing a novel perspective on the sol–gel synthesis of NVPF cathodes.


 Please wait while we load your content...
Please wait while we load your content...