The role of the pre-exponential factor in determining the kinetic selection of polymorphs during solution crystallization of organic compounds
Abstract
Generally, pairs of polymorphs can be characterized by their ratios of equilibrium solubilities 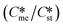 and interfacial energies (γst/γme) for a given temperature and solvent. We refer to this point as the solubility-interfacial energy characteristic point (characteristic point for short) of a polymorphic pair. The equations of the classical nucleation theory have been used to determine the influence of supersaturation, the absolute size of the interfacial energies and the ratio of the pre-exponential factors for pairs of polymorphs to predict the experimental conditions in which metastable or stable polymorphs crystallize first. Domain diagrams for polymorph pairs based on the equilibrium solubility ratios
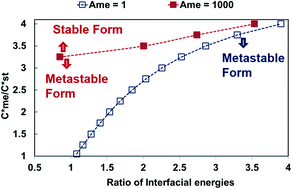
and interfacial energies (γst/γme) for a given temperature and solvent. We refer to this point as the solubility-interfacial energy characteristic point (characteristic point for short) of a polymorphic pair. The equations of the classical nucleation theory have been used to determine the influence of supersaturation, the absolute size of the interfacial energies and the ratio of the pre-exponential factors for pairs of polymorphs to predict the experimental conditions in which metastable or stable polymorphs crystallize first. Domain diagrams for polymorph pairs based on the equilibrium solubility ratios 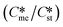 and the ratio of interfacial energies (γst/γme) have been developed. Separate zones are identified where the metastable and stable polymorphs are favoured kinetically; generally higher supersaturation kinetically favour the metastable form. This contribution investigates the circumstances where large values for the pre-exponential factor, particularly for the metastable polymorph, in the classical nucleation theory description of nucleation can expand the zone where the metastable zone is kinetically favoured. The results indicate that the pre-exponential factor has a strong influence in expanding the kinetically metastable zone when the interfacial energies of the metastable and stable polymorphic are low (less than 3.5 mJ m−2) but has little or no effect when these values are high (greater than 5.5 mJ m−2). This work also identifies the circumstances where a metastable polymorph with a higher interfacial energy than the stable polymorph will crystallize first.
and the ratio of interfacial energies (γst/γme) have been developed. Separate zones are identified where the metastable and stable polymorphs are favoured kinetically; generally higher supersaturation kinetically favour the metastable form. This contribution investigates the circumstances where large values for the pre-exponential factor, particularly for the metastable polymorph, in the classical nucleation theory description of nucleation can expand the zone where the metastable zone is kinetically favoured. The results indicate that the pre-exponential factor has a strong influence in expanding the kinetically metastable zone when the interfacial energies of the metastable and stable polymorphic are low (less than 3.5 mJ m−2) but has little or no effect when these values are high (greater than 5.5 mJ m−2). This work also identifies the circumstances where a metastable polymorph with a higher interfacial energy than the stable polymorph will crystallize first.


 Please wait while we load your content...
Please wait while we load your content...