A readily synthesized bismuth oxyiodide/attapulgite for the photodegradation of tetracycline under visible light irradiation
Abstract
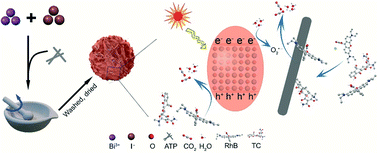
Bismuth oxyiodide and attapulgite have proven to be potential materials for the removal of emerging contaminants in wastewater. In this work, bismuth oxyiodide/attapulgite (BIA-x) composites with various amounts of attapulgite were readily synthesized by mechanical grinding in ta mortar at room temperature. The chemical bonds, specific surface area, and morphology of resulting materials were characterized by Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, surface area, field emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, and transmission electron microscopy. In the photodegradation process, BIA-8 exhibits the best performance for both rhodamine B (96.3% removal in 40 min, 4.22-fold as BiOI) and tetracycline (85.2% removal in 40 min, 1.67-fold as BiOI) removal. Furthermore, photoelectrochemical and radical trapping experiments were performed to study the mechanisms of photodegradation. Based on the experimental results, holes, and superoxide radicals were the main active species during the photodegradation possess. The hydroxyl radicals were not adequate to acquire due to the low valence band position. The photodegradation capacity increased after attapulgite was added, which might be due to the synergism of bismuth oxyiodide and attapulgite.


 Please wait while we load your content...
Please wait while we load your content...