The solvent influenced coordination variation of flexible ligands to Y(iii) towards MOF structural diversity†
Abstract
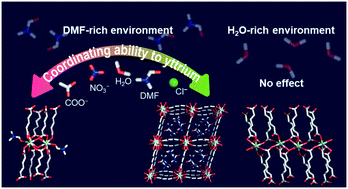
The formation of coordination complexes, including metal–organic frameworks, occurs following the order of coordinating ability, which is the order of preference for molecules or ions to coordinate to a specific metal. The coordinating ability helps to predict and understand the resulting structures when it provides an absolute rule. Unfortunately, it frequently fails to follow a fixed order due to various environmental reaction parameters. We show that the conventional coordinating ability to yttrium becomes non-applicable in a H2O-rich solvent environment, resulting in compounds with the same structure from Y(NO3)3 and YCl3 precursors, while the order works well in a DMF-rich environment to give two compounds with different structures from Y(NO3)3 and YCl3 precursors. As a result, overall, three different yttrium-based MOF structures have been newly obtained from a single metal and ligand set, i.e., Y(III) and muconic acid through controlling the coordinating abilities of Y(III) via simply modulating the solvent environment: [Y(muco)1.5(H2O)] (1), [Y(muco)1.5(DMF)] (2), and [Y(muco)1.5(H2O)2]·2DMF (3). Our findings provide insight into the influence of the solvent environment on the role of the coordinating ability for the formation of yttrium coordination compounds and Y-MOFs with diverse structures.


 Please wait while we load your content...
Please wait while we load your content...